Thymagen (Thymogen) is a highly active bioregulator peptide with primary effects on cells of the immune system.
$80 USD
Out of stock
| Expiry date | 3/12/27 |
| Date Produced | 3/12/25 |
| CAS # | 38101-59-6 |
| Formula | C₁₆H₁₉N₃O₅ |
| M.W. | 333.34 g/mol |
| REF | 100094 |
| Purity | 99% HPLC |
| RUO | Research Use Only |
Not for human or veterinary use. Made in USA
This product is intended as a research chemical only. Not for human use. Peptides will arrive in a lyophilized (powder) form for maximum stability.
Thymagen (Thymogen) is a dipeptide bioregulator with primary effects on the thymus and immune system. Originally isolated from calf thymus, Thymagen is now produced via recombinant DNA techniques. Research shows that it can stimulate the immune response to a number of viruses and a variety of bacterial infections.
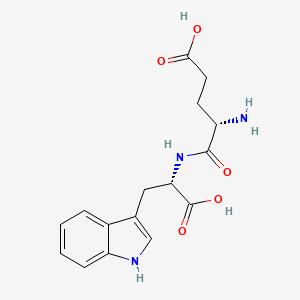
Amino Acid Sequence: Glu-Trp (EW)
Molecular Formula: C₁₆H₁₉N₃O₅
Molecular Weight: 333.34 g/mol
PubChem CID: 100094
CAS Number: 38101-59-6
Synonyms: Oglufanide, Thymogen
Cyclic nucleotides are single-phosphate nucleotides with a cyclic bond arrangement between the sugar and phosphate groups. They are integral components of communication within cells, usually acting as second messengers within the cell after a protein on the cell surface has bound to something. In other words, cyclic nucleotides act as messengers within cells for substances that cannot enter the cells themselves.
Research on Thymagen shows that it downregulates cyclic nucleotide catabolism. In other words, Thymagen slows the breakdown of cyclic nucleotides and thereby raises their levels within the cell. This results in enhanced ability of cells, particularly those of the immune system, to respond to messages from other parts of the body. For example, increased levels of cyclic nucleotides could make cells of the immune system more responsive to invading pathogens by improving signaling between these cells.
The above literature was researched, edited and organized by Dr. E. Logan, M.D. Dr. E. Logan holds a doctorate degree from Case Western Reserve University School of Medicine and a B.S. in molecular biology.
Vladimir Khavinson is a Professor, President of the European region of the International Association of Gerontology and Geriatrics; Member of the Russian and Ukrainian Academies of Medical Sciences; Main gerontologist of the Health Committee of the Government of Saint Petersburg, Russia; Director of the Saint Petersburg Institute of Bioregulation and Gerontology; Vice-president of the Gerontological Society of the Russian Academy of Sciences; Head of the Chair of Gerontology and Geriatrics of the North-Western State Medical University, St. Petersburg; Colonel of medical service (USSR, Russia), retired.
Vladimir Khavinson is known for the discovery, experimental, and clinical studies of new classes of peptide bioregulators as well as for the development of bioregulating peptide therapy. He is engaged in studying the role of peptides in the regulation of ageing mechanisms. His main field of work includes the design, pre-clinical, and clinical studies of new peptide geroprotectors.
A 40-year-long investigation resulted in a multitude of methods for applying peptide bioregulators to slow the ageing process and increase human lifespan. Six peptide-based pharmaceuticals and 64 peptide food supplements have been introduced into clinical practice by V. Khavinson. He is the author of 196 patents (Russian and international) and 775 scientific publications. His major achievements are presented in two books: “Peptides and Ageing” (NEL, 2002) and “Gerontological Aspects of Genome Peptide Regulation” (Karger AG, 2005).
Vladimir Khavinson introduced the scientific specialty “Gerontology and Geriatrics” at the governmental level in the Russian Federation. The Academic Council headed by V. Khavinson has overseen more than 200 Ph.D. and Doctorate theses from many countries.
S. V. Demidov, A. N. Kostromin, V. V. Kuĭbeda, I. V. Chernaia, and M. I. Borovok, “Effect of thymagen, thymalin and vilosen on the cAMP and cGMP levels and phosphodiesterase activity in spleen lymphocytes during sensitization and anaphylactic shock,” Ukr. Biokhimicheskii Zhurnal 1978, vol. 63, no. 4, pp. 104–106, Aug. 1991.
A. L. Kozhemiakin, V. G. Morozov, and V. K. Khavinson, “Participation of the cyclase system in the molecular mechanisms of differentiation control of immunocompetent cells,” Biokhimiia Mosc. Russ., vol. 49, no. 4, pp. 658–666, Apr. 1984.
D. S. Silin, O. V. Lyubomska, F. I. Ershov, V. M. Frolov, and G. A. Kutsyna, “Synthetic and natural immunomodulators acting as interferon inducers,” Curr. Pharm. Des., vol. 15, no. 11, pp. 1238–1247, 2009. doi: 10.2174/138161209787846847.
N. D. Iushchuk, G. I. Tseneva, T. V. Alenushkina, and L. B. Kuliashova, “The efficacy of using thymogen in an experimental infection caused by Yersinia enterocolitica,” Zh. Mikrobiol. Epidemiol. Immunobiol., no. 3, pp. 106–108, Jun. 1995.
E. A. Zhuk and V. A. Galenok, “Thymogen in the treatment of type-1 diabetes mellitus,” Ter. Arkh., vol. 68, no. 10, pp. 12–14, 1996.
O. K. Khmel’nitskiĭ, G. M. Iakovlev, V. L. Belianin, V. K. Khavinson, V. G. Morozov, and V. I. Deĭgin, “The effect of a synthetic thymus peptide (thymogen) on the immune system in candidiasis under immunodepression,” Arkh. Patol., vol. 52, no. 1, pp. 20–25, 1990.
V. S. Smirnov, S. V. Petlenko, and S. S. El’tsin, “Application thymogen for preoperative preparation of elderly patients with tumor processes in abdominal cavity,” Adv. Gerontol. Uspekhi Gerontol., vol. 24, no. 2, pp. 278–284, 2011.
K. M. Reznikov, O. V. Vinokurova, V. V. Alabovskiĭ, and A. A. Vinokurov, “The anti-arrhythmia properties of thymogen,” Eksp. Klin. Farmakol., vol. 57, no. 6, pp. 31–33, Dec. 1994.
O. V. Filippova, K. M. Reznikov, V. V. Alabovskił, V. V. Khamburov, and A. A. Vinokurov, “The effect of thymogen on the heart in ischemia and reperfusion,” Eksp. Klin. Farmakol., vol. 60, no. 3, pp. 27–29, Jun. 1997.
V. N. Anisimov, G. I. Miretskiĭ, V. G. Morozov, I. A. Pavel’eva, and V. K. Khavinson, “The effect of the synthetic immunomodulator thymogen on radiation-induced carcinogenesis in rats,” Vopr. Onkol., vol. 38, no. 4, pp. 451–458, 1992.
V. G. Bespalov, D. N. Troian, A. S. Petrov, V. G. Morozov, and V. K. Khavinson, “Inhibiting effect of thymogen on the development of tumors of the esophagus and forestomach induced by N-nitrososarcosine ethyl ester in rats,” Eksp. Onkol., vol. 11, no. 4, pp. 23–26, 1989.
All of our products are manufactured using the Lyophilization (Freeze Drying) process, which ensures that our products remain 100% stable for shipping for up to 3-4 months.
Once the peptides are reconstituted (mixed with bacteriostatic water), they must be stored in the fridge to maintain stability. After reconstitution, the peptides will remain stable for up to 30 days.
Lyophilization is a unique dehydration process, also known as cryodesiccation, where the peptides are frozen and then subjected to low pressure. This causes the water in the peptide vial to sublimate directly from solid to gas, leaving behind a stable, crystalline white structure known as lyophilized peptide. The puffy white powder can be stored at room temperature until you’re ready to reconstitute it with bacteriostatic water.
Once peptides have been received, it is imperative that they are kept cold and away from light. If the peptides will be used immediately, or in the next several days, weeks or months, short-term refrigeration under 4C (39F) is generally acceptable. Lyophilized peptides are usually stable at room temperatures for several weeks or more, so if they will be utilized within weeks or months such storage is typically adequate.
However, for longer term storage (several months to years) it is more preferable to store peptides in a freezer at -80C (-112F). When storing peptides for months or even years, freezing is optimal in order to preserve the peptide’s stability.
For further information on proper storage techniques, click the link below:
Peptide Storage Information
This product is intended as a research chemical only. Not for human use. Peptides will arrive in a lyophilized (powder) form for maximum stability.
Thymagen (Thymogen) is a dipeptide bioregulator with primary effects on the thymus and immune system. Originally isolated from calf thymus, Thymagen is now produced via recombinant DNA techniques. Research shows that it can stimulate the immune response to a number of viruses and a variety of bacterial infections.

Amino Acid Sequence: Glu-Trp (EW)
Molecular Formula: C₁₆H₁₉N₃O₅
Molecular Weight: 333.34 g/mol
PubChem CID: 100094
CAS Number: 38101-59-6
Synonyms: Oglufanide, Thymogen
Cyclic nucleotides are single-phosphate nucleotides with a cyclic bond arrangement between the sugar and phosphate groups. They are integral components of communication within cells, usually acting as second messengers within the cell after a protein on the cell surface has bound to something. In other words, cyclic nucleotides act as messengers within cells for substances that cannot enter the cells themselves.
Research on Thymagen shows that it downregulates cyclic nucleotide catabolism. In other words, Thymagen slows the breakdown of cyclic nucleotides and thereby raises their levels within the cell. This results in enhanced ability of cells, particularly those of the immune system, to respond to messages from other parts of the body. For example, increased levels of cyclic nucleotides could make cells of the immune system more responsive to invading pathogens by improving signaling between these cells.
The above literature was researched, edited and organized by Dr. E. Logan, M.D. Dr. E. Logan holds a doctorate degree from Case Western Reserve University School of Medicine and a B.S. in molecular biology.
Vladimir Khavinson is a Professor, President of the European region of the International Association of Gerontology and Geriatrics; Member of the Russian and Ukrainian Academies of Medical Sciences; Main gerontologist of the Health Committee of the Government of Saint Petersburg, Russia; Director of the Saint Petersburg Institute of Bioregulation and Gerontology; Vice-president of the Gerontological Society of the Russian Academy of Sciences; Head of the Chair of Gerontology and Geriatrics of the North-Western State Medical University, St. Petersburg; Colonel of medical service (USSR, Russia), retired.
Vladimir Khavinson is known for the discovery, experimental, and clinical studies of new classes of peptide bioregulators as well as for the development of bioregulating peptide therapy. He is engaged in studying the role of peptides in the regulation of ageing mechanisms. His main field of work includes the design, pre-clinical, and clinical studies of new peptide geroprotectors.
A 40-year-long investigation resulted in a multitude of methods for applying peptide bioregulators to slow the ageing process and increase human lifespan. Six peptide-based pharmaceuticals and 64 peptide food supplements have been introduced into clinical practice by V. Khavinson. He is the author of 196 patents (Russian and international) and 775 scientific publications. His major achievements are presented in two books: “Peptides and Ageing” (NEL, 2002) and “Gerontological Aspects of Genome Peptide Regulation” (Karger AG, 2005).
Vladimir Khavinson introduced the scientific specialty “Gerontology and Geriatrics” at the governmental level in the Russian Federation. The Academic Council headed by V. Khavinson has overseen more than 200 Ph.D. and Doctorate theses from many countries.
S. V. Demidov, A. N. Kostromin, V. V. Kuĭbeda, I. V. Chernaia, and M. I. Borovok, “Effect of thymagen, thymalin and vilosen on the cAMP and cGMP levels and phosphodiesterase activity in spleen lymphocytes during sensitization and anaphylactic shock,” Ukr. Biokhimicheskii Zhurnal 1978, vol. 63, no. 4, pp. 104–106, Aug. 1991.
A. L. Kozhemiakin, V. G. Morozov, and V. K. Khavinson, “Participation of the cyclase system in the molecular mechanisms of differentiation control of immunocompetent cells,” Biokhimiia Mosc. Russ., vol. 49, no. 4, pp. 658–666, Apr. 1984.
D. S. Silin, O. V. Lyubomska, F. I. Ershov, V. M. Frolov, and G. A. Kutsyna, “Synthetic and natural immunomodulators acting as interferon inducers,” Curr. Pharm. Des., vol. 15, no. 11, pp. 1238–1247, 2009. doi: 10.2174/138161209787846847.
N. D. Iushchuk, G. I. Tseneva, T. V. Alenushkina, and L. B. Kuliashova, “The efficacy of using thymogen in an experimental infection caused by Yersinia enterocolitica,” Zh. Mikrobiol. Epidemiol. Immunobiol., no. 3, pp. 106–108, Jun. 1995.
E. A. Zhuk and V. A. Galenok, “Thymogen in the treatment of type-1 diabetes mellitus,” Ter. Arkh., vol. 68, no. 10, pp. 12–14, 1996.
O. K. Khmel’nitskiĭ, G. M. Iakovlev, V. L. Belianin, V. K. Khavinson, V. G. Morozov, and V. I. Deĭgin, “The effect of a synthetic thymus peptide (thymogen) on the immune system in candidiasis under immunodepression,” Arkh. Patol., vol. 52, no. 1, pp. 20–25, 1990.
V. S. Smirnov, S. V. Petlenko, and S. S. El’tsin, “Application thymogen for preoperative preparation of elderly patients with tumor processes in abdominal cavity,” Adv. Gerontol. Uspekhi Gerontol., vol. 24, no. 2, pp. 278–284, 2011.
K. M. Reznikov, O. V. Vinokurova, V. V. Alabovskiĭ, and A. A. Vinokurov, “The anti-arrhythmia properties of thymogen,” Eksp. Klin. Farmakol., vol. 57, no. 6, pp. 31–33, Dec. 1994.
O. V. Filippova, K. M. Reznikov, V. V. Alabovskił, V. V. Khamburov, and A. A. Vinokurov, “The effect of thymogen on the heart in ischemia and reperfusion,” Eksp. Klin. Farmakol., vol. 60, no. 3, pp. 27–29, Jun. 1997.
V. N. Anisimov, G. I. Miretskiĭ, V. G. Morozov, I. A. Pavel’eva, and V. K. Khavinson, “The effect of the synthetic immunomodulator thymogen on radiation-induced carcinogenesis in rats,” Vopr. Onkol., vol. 38, no. 4, pp. 451–458, 1992.
V. G. Bespalov, D. N. Troian, A. S. Petrov, V. G. Morozov, and V. K. Khavinson, “Inhibiting effect of thymogen on the development of tumors of the esophagus and forestomach induced by N-nitrososarcosine ethyl ester in rats,” Eksp. Onkol., vol. 11, no. 4, pp. 23–26, 1989.
All of our products are manufactured using the Lyophilization (Freeze Drying) process, which ensures that our products remain 100% stable for shipping for up to 3-4 months.
Once the peptides are reconstituted (mixed with bacteriostatic water), they must be stored in the fridge to maintain stability. After reconstitution, the peptides will remain stable for up to 30 days.
Lyophilization is a unique dehydration process, also known as cryodesiccation, where the peptides are frozen and then subjected to low pressure. This causes the water in the peptide vial to sublimate directly from solid to gas, leaving behind a stable, crystalline white structure known as lyophilized peptide. The puffy white powder can be stored at room temperature until you’re ready to reconstitute it with bacteriostatic water.
Once peptides have been received, it is imperative that they are kept cold and away from light. If the peptides will be used immediately, or in the next several days, weeks or months, short-term refrigeration under 4C (39F) is generally acceptable. Lyophilized peptides are usually stable at room temperatures for several weeks or more, so if they will be utilized within weeks or months such storage is typically adequate.
However, for longer term storage (several months to years) it is more preferable to store peptides in a freezer at -80C (-112F). When storing peptides for months or even years, freezing is optimal in order to preserve the peptide’s stability.
For further information on proper storage techniques, click the link below:
Peptide Storage Information
From $43 USD - $50 USD
From $43 USD - $50 USD
From $43 USD - $50 USD
From $43 USD - $50 USD
From $43 USD - $50 USD
From $85 USD - $100 USD
Receive all the latest information on events, sales, & offers.

Mon - Fri 9AM - 4PM PST
Mon - Fri (Excluding holidays)
Orders placed and paid after 12PM PST are shipped the following business day
© 2025
Ai-Peptides.com. All Rights Reserved.
All products on this site are for Research, Development use only. Products are Not for Human consumption of any kind.
The statements made within this website have not been evaluated by the US Food and Drug Administration. The statements and the products of this company are not intended to diagnose, treat, cure or prevent any disease.
Ai-Peptides is a chemical supplier. Ai-Peptides is not a compounding pharmacy or chemical compounding facility as defined under 503A of the Federal Food, Drug, and Cosmetic act. AiPeptides is not an outsourcing facility as defined under 503B of the Federal Food, Drug, and Cosmetic act.