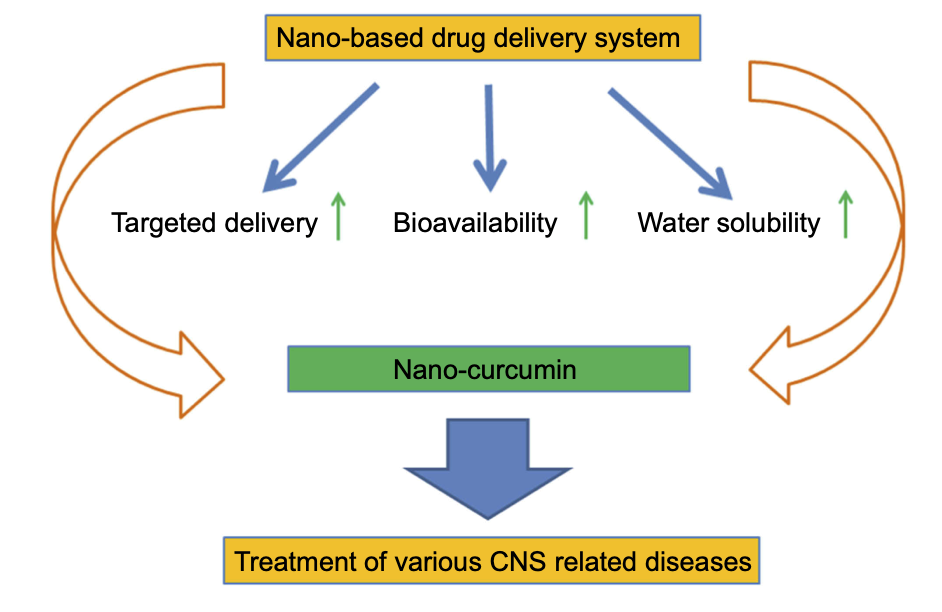
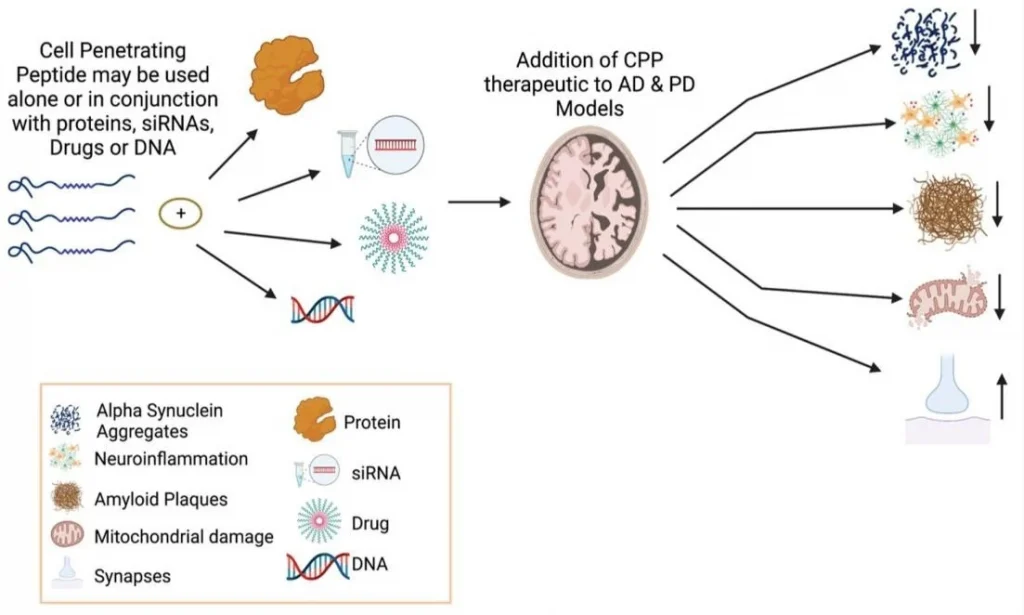
Figure 1: Neuroprotective features of CPPs in combination with other molecules or peptides for treating PD and AD.
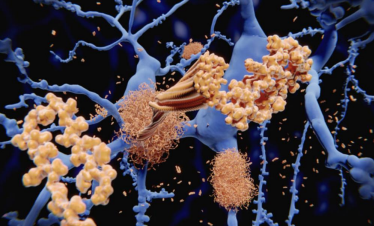
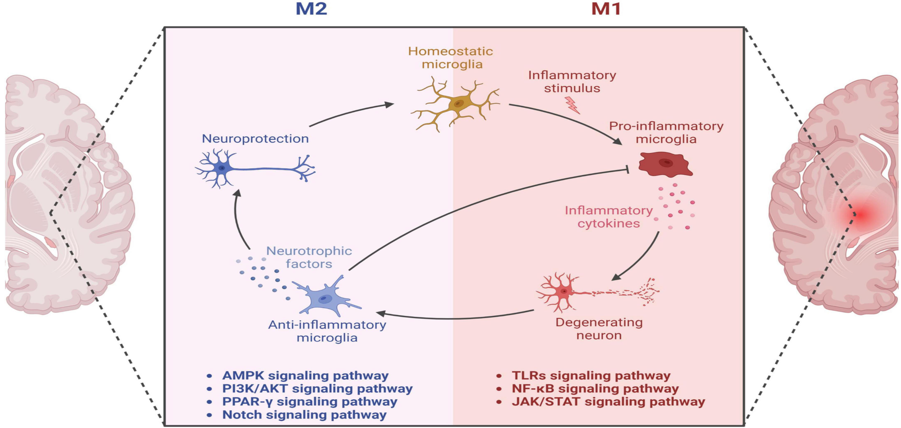
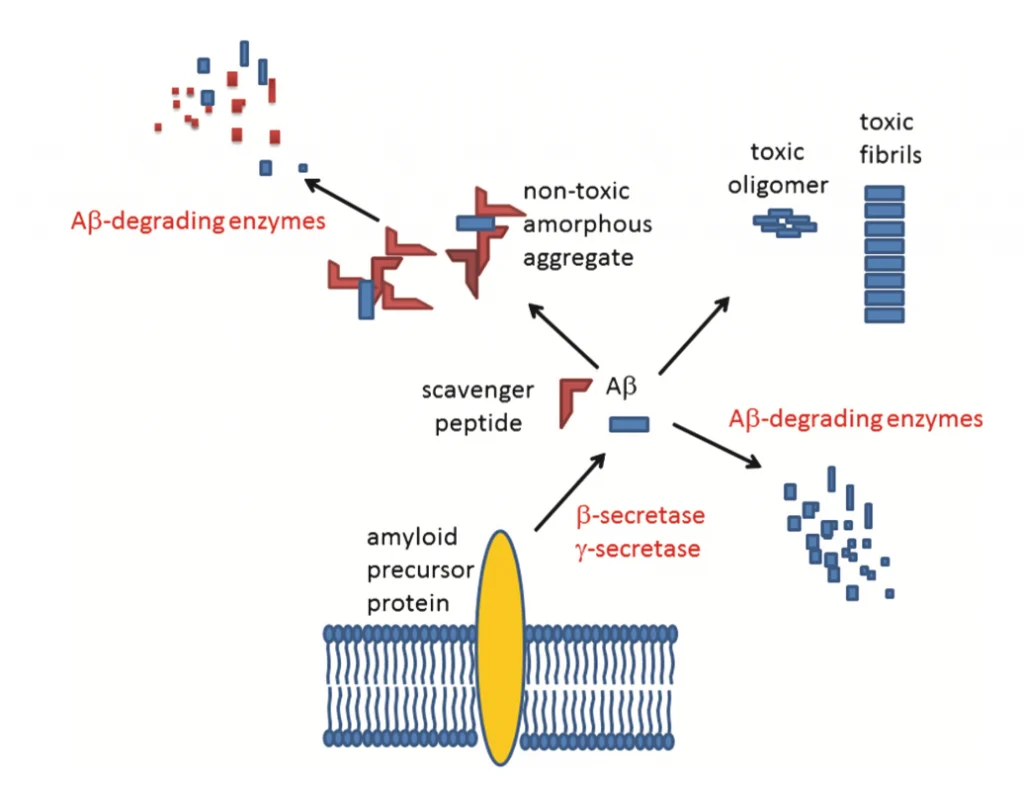
JNK family participates in the apoptosis pathway, also known as programmed cell death.[4] The apoptosis mechanism is used to eliminate cells that are damaged in anyway. This mechanism is suitable for healthy people because it helps avoid developing cancer and other disorders.[1] On the other hand, apoptosis is detrimental in neurodegenerative diseases that share the abnormal accumulation of misfolded proteins, contributing to dementia, cognitive loss, memory loss, behavioral problems, and sleep problems, among others, through neuronal cell loss.[3,5,7] In addition, the JNK family also participates in pathways related to regulation, plasticity, development of the Central Nervous System (CNS), inflammation, and autophagy.[9] The well-functioning of all these processes is vital. Recent studies suggested that an imbalance of the JNK family members can accelerate the progression of both AD and PD pathologies. One of the hallmarks of AD is the aggregation of amyloid beta (Aβ) in the extracellular area.[7] The overexpression of Aβ triggers the activation of JNK-3, which also participates in the formation of Aβ42, a toxic species that affects the neuron’s cell function when it accumulates.[7] It is unclear whether the neurodegenerative disorder or the JNK imbalance comes first. However, they both have a direct relationship where PD and AD patients show high levels of JNK in the brain (postmortem), and irregular levels of the JNK family accelerate the progression of both disorders (see Fig. 2). [5,9] JNK imbalance could also lead to increased inflammation responses, low plasticity, and a flaw in autophagy performance, among other related adverse effects. An increase in apoptosis in PD and AD patients causes a decrease in neurons in the brain, affecting cognitive, behavioral, and memory performance.[8] JNK also decreases the α-syn accumulation in PD models, contributing to neuroprotection.[10] JNK pathway influence positively many vital processes of the body unless an imbalance occurs. [2,8] JNK has been the target for treating several diseases like cancer, strokes, PD, AD, Huntington’s disease, and other neurodegenerative disorders, showing promising results. More investigations need to be done to comprehend better how the correct levels of JNK can help combat important hallmarks of degenerative illnesses to eventually prevent or revert the progression of related diseases like PD and AD. In general, the benefits of inhibiting JNKs for treating neurodegenerative disorders are:
Improving autophagy for the elimination of misfolded proteins (Aβ (AD), tau protein (AD), and α-syn (PD)
- Increasing cell proliferation.
- Decreasing programmed cell death.
- Reducing brain damage.
- Decreasing cognitive decline.
- Increasing gene expression.
- Decreasing neuroinflammation responses
- Improving memory
- Providing neuroprotection
- Improving synaptic connections