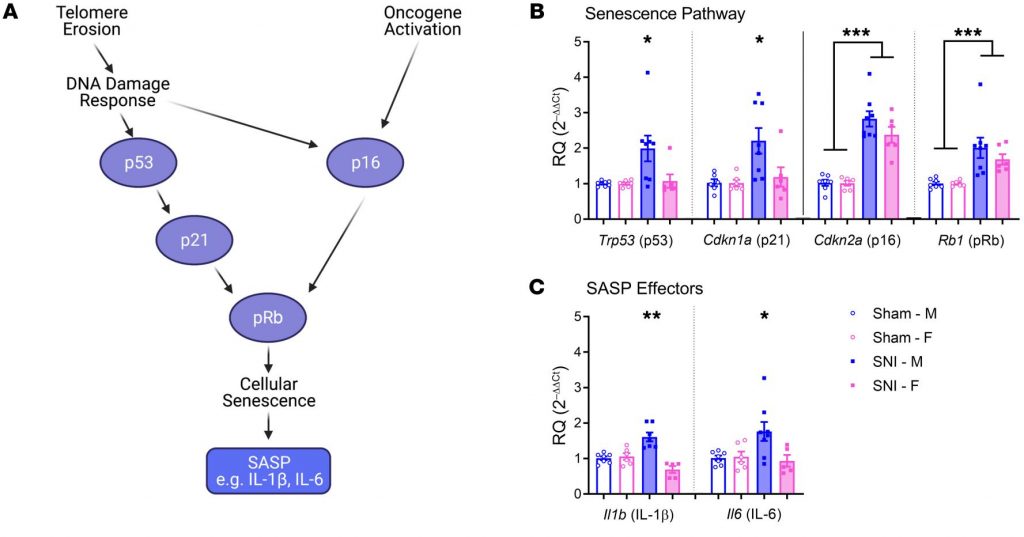
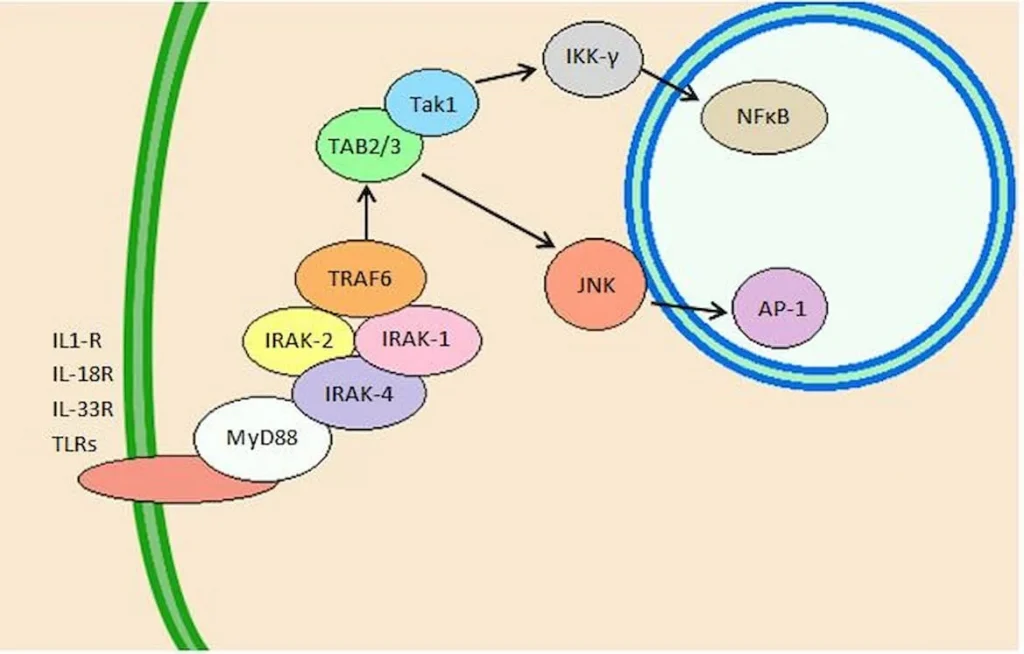
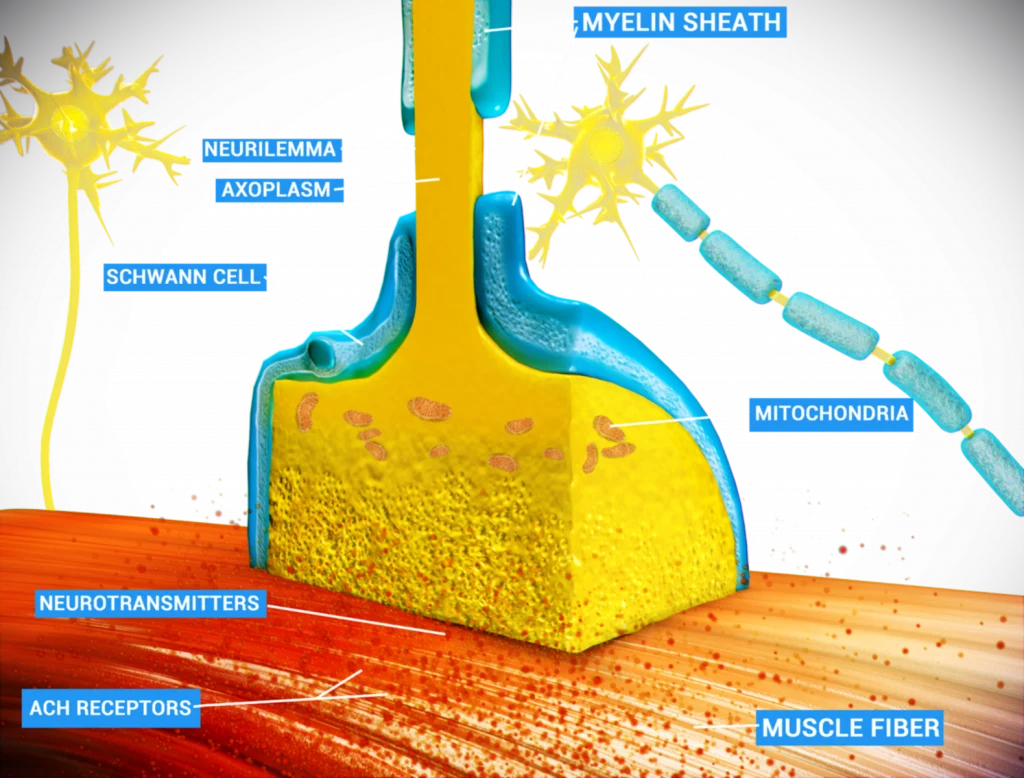
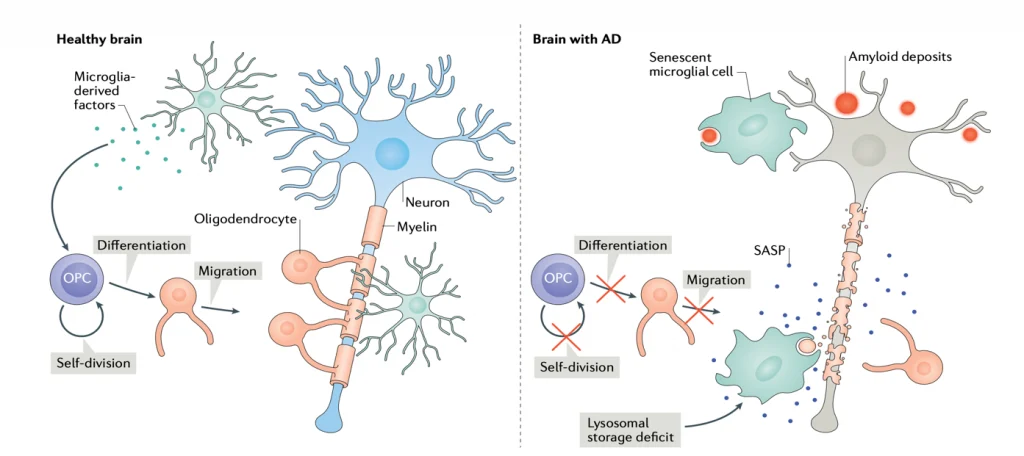
When Dead Cells Don’t Get Cleaned Up, Diseases Emerge:
“This process, termed efferocytosis, is critical for the prevention of autoimmune and inflammatory disorders, and persistence of dead cells in tissue is characteristic of many human autoimmune diseases, notably systemic lupus erythematosus.” (3)“Aberrations in efferocytosis are associated with numerous inflammatory pathologies, including atherosclerosis, cancer and infections. The recent exciting discoveries defining the molecular machinery involved in efferocytosis have opened many avenues for therapeutic intervention, with several agents now in clinical trials.” (5)
Definition of Efferocytosis:
“In cell biology, efferocytosis is the process by which apoptotic cells are removed by phagocytic cells. It can be regarded as the ‘burying of dead cells’.” (1)
“Efferocytosis is a term derived from the Greek (meaning to carry the dead to the grave), and refers to the phagocytic engulfment of a cellular corpse. Both professional phagocytes (e.g. macrophages) and non-professional (e.g. neighboring) cells participate in efferocytosis.” (2)
Efferocytosis In Depth, The 4 Stages: