Thymosin-β4, and Human Vitronectin peptides Grafted to Collagen Tune Adhesion or VEGF Gene Expression in Human Cell Lines
Collagen is the most abundant protein in the human body, found in the bones, muscles, skin, and tendons. It provides structural support to the extracellular space of connective tissues. Due to its rigidity and resistance to stretching, it is the perfect matrix for skin, tendons, bones, and ligaments.
Researchers wanted to test the modulation of adhesiveness strength and specificity of collagen scaffolds through the grafting of adhesive peptides. They thought that this may improve both cell adhesion and migration, favoring the tissue regenerative process. However, to date, no such study has been performed.
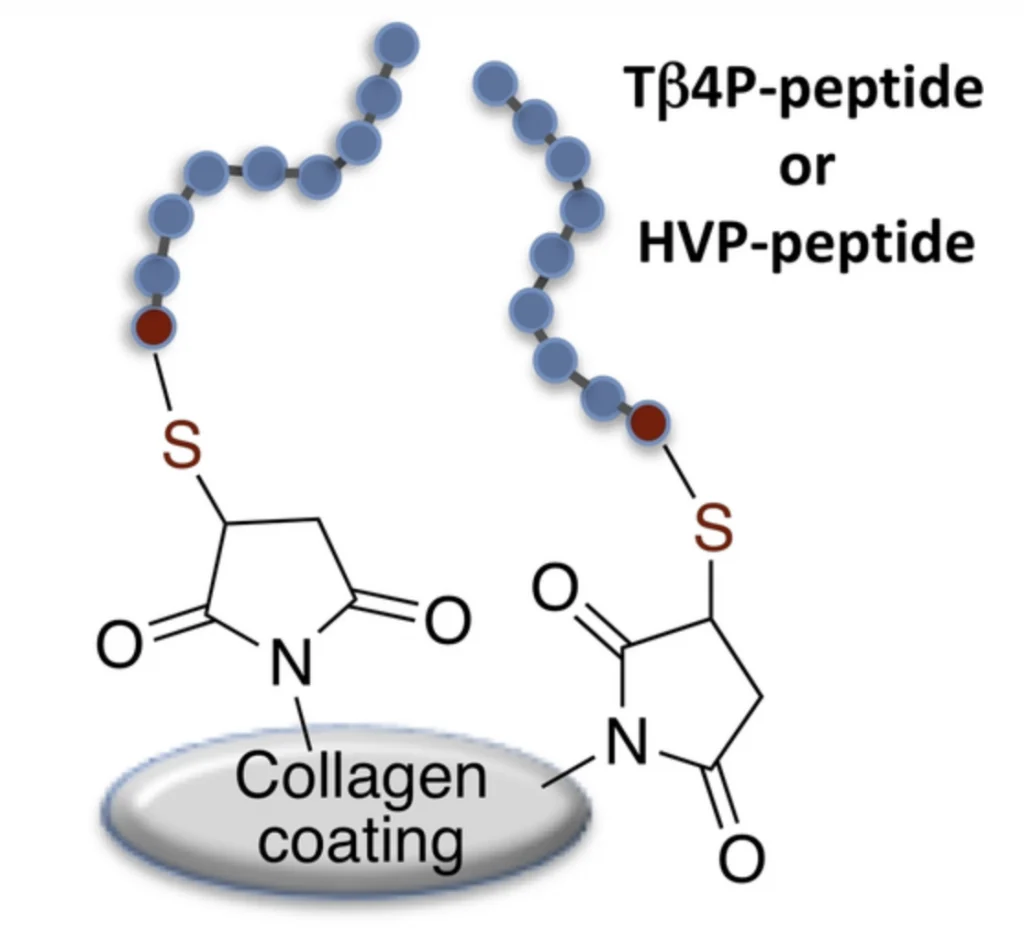
More specifically, in the study, researchers examined thymosin-β4 (Tβ4P) and Human Vitronectin (HVP) (seen in the image to the right) derived peptides grafted to collagen by thiolene Michael addition in order to improve collagen bioactivity for regenerative medicine approaches.
Tβ4P and HVP are known to exert proangiogenic and proadhesive activity respectively, and HVP is involved in osteogenesis promotion. The ability of these peptides to increase collagen cell adhesion and angiogenesis properties is assessed on human cell lines. See image below showing the peptides grafting strategy to collagen coatings.