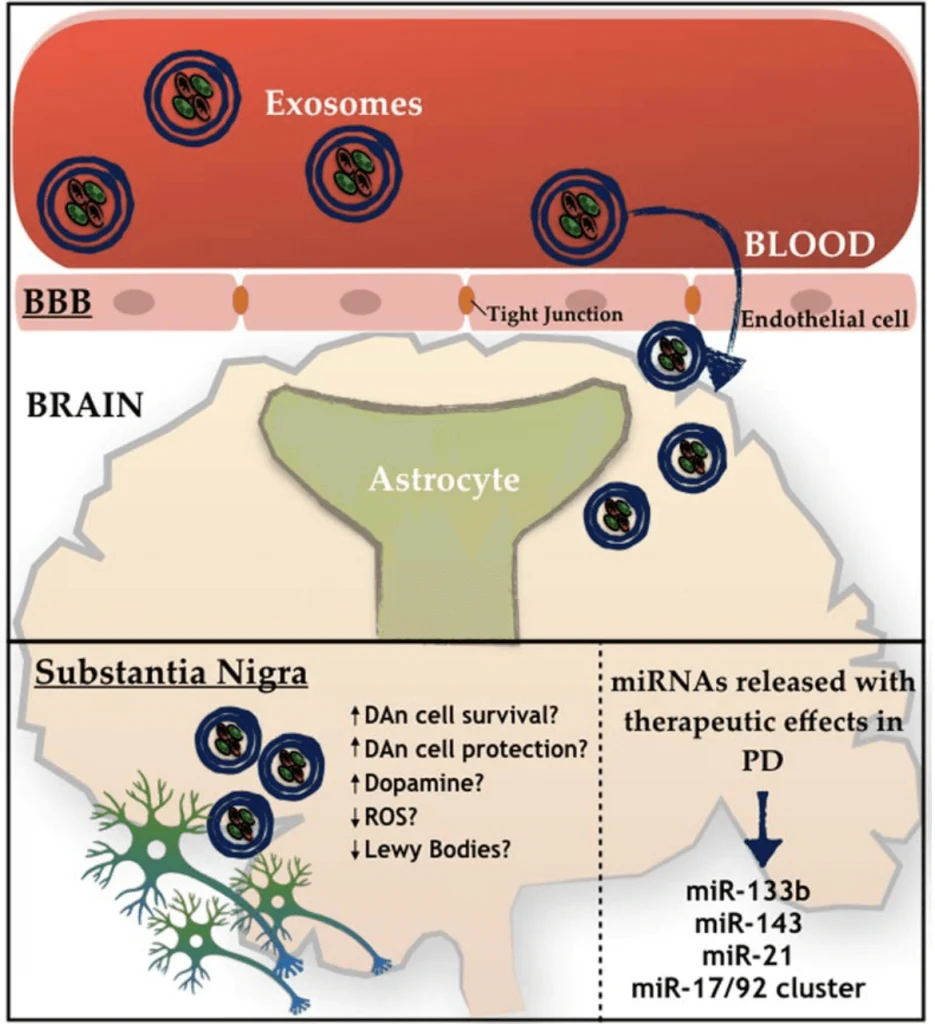
Thymosin Beta-4
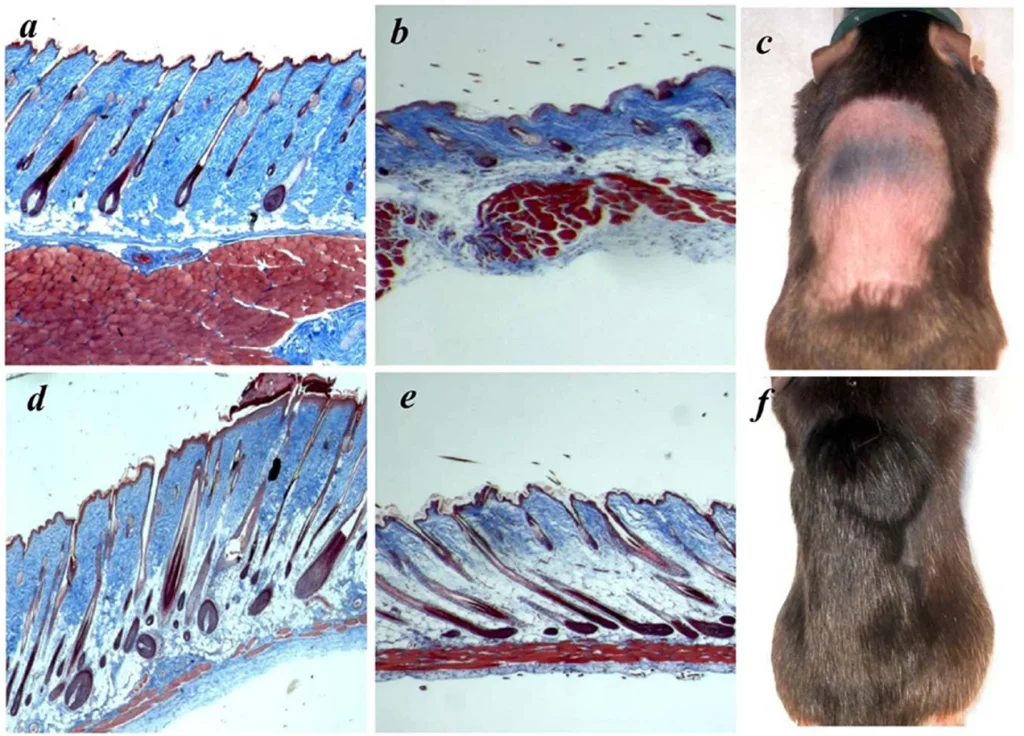
Thymosin Beta 4, is a member of a highly conserved family of actin monomer-sequestering proteins. Thymosin β-4 is a 43 amino acid sequence encoded by gene TMSBX4 present in all human cells. It is naturally found in higher concentrations in tissue damaged areas and has been frequently used in sports doping for the past 20 years for its ability to decrease injury times and reduce delayed onset muscle soreness. TB-4 has been used for soft tissue repair (this includes tendon, ligament, and muscle), sports and athletic injuries, pressure or venous stasis ulcers, immune response modulation, brain issues related to autoimmunity, and spinal cord injuries. In addition to its role as a major actin-sequestering molecule, Thymosin Beta 4 plays a role in tissue repair. Tβ4 has been found to play an important role in protection, regeneration and remodeling of injured or damaged tissues. The gene for Tβ4 has also been found to be one of the first to be upregulated after injuries. Thymosin Beta 4 is most often prescribed for acute injury, surgical repair and for senior athletes. It has most recently been shown to help regrow hair in addition to PRP and has several effects on stem cell activation.
Advances in the basic and clinical applications of thymosin beta 4:
Based on its multifunctional activities during tissue regeneration in various animal studies in this report, Tb4 has the potential for new clinical applications such kidney and liver disease, as well as repair of spinal cord, bone and ligament damage.
The effect on stem cells:
This study shows that Tβ4 promotes the expansion of human ASCs via an IL-8-dependent mechanism that involves the ERK and NF-κB pathways. Therefore, Tβ4 could be used as a tool for MSC expansion in cell therapeutics.
Tb4 initiated cell fate determination of MSCs through biophysical effects exerted by cytoskeleton reorganization and altered cell-cell adhesion rather than direct regulation of lineage-determining transcription factors.
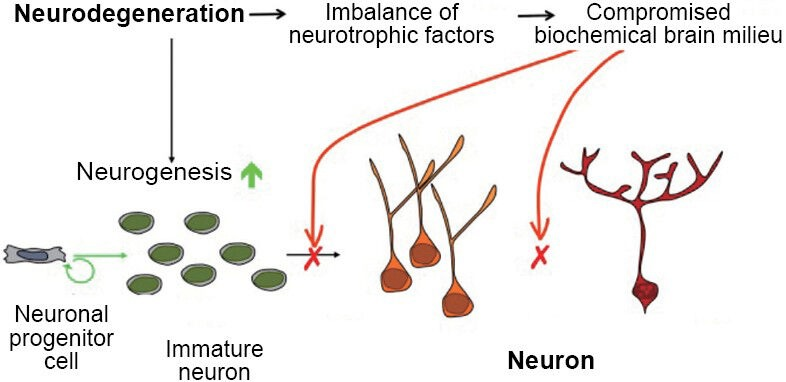
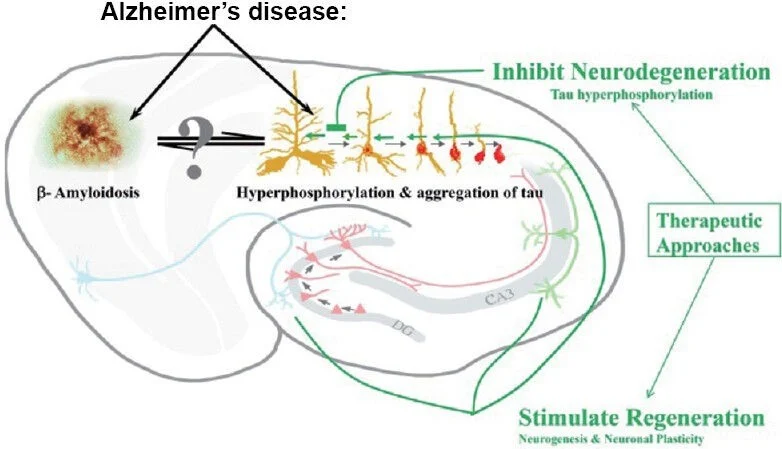
Delayed (24 hours post injury) Tβ4 treatment promotes neurogenesis after TBI in rats.
The miR-200 not only induces terminal differentiation of NPCs in the OB, but also induces neuronal differentiation in neuronal stem cells and in a PC-12 cell line.
PEG-MGF
MGF is a split variant of IGF-1 but its sequence differs from the systemic IGF-1 produced by the liver. MGF initiates hypertrophy and repair of local muscle damage. MGF is expressed by mechanically overloaded muscle and is involved in tissue repair and adaptation. It is expressed as a pulse following muscle damage and is involved in the activation of muscle satellite (stem) cells. These donate nuclei to the muscle fibers that are required for repair and for the hypertrophy process, which may have similar regulatory mechanisms. MGF is essential for repair and therefore growth of new cells, similar to IGF-1.
The effect on stem cells:
Both wound-healing and transwell assays indicated that MGF E peptide could significantly enhance rBMSCs migration ability.