Programmed cell death, better known as apoptosis, is crucial in eliminating abnormal or unwanted cells in the body.[2] In recent years, the complex pathways regulating apoptosis have been well-studied as potential therapy targets.[2,10] Parkinson’s disease (PD) is no exception since the leading cause of this disorder is the death of dopamine-producing neurons in the brain (see Fig.1).[1-4] PD is a debilitating neurodegenerative disorder that steals a person’s ability to control movement.[8] Currently, levodopa (L-Dopa) is the most effective treatment for PD, helping in the improvement of the symptoms but not in the progression of the disease.[3] In addition, the use of L-Dopa led to adverse reactions after long-term administration.[3,7] Several studies propose using microRNAs (miRNAs) to inhibit the apoptosis of the dopamine-producing neurons found in PD.[6-9] miRNAs are tiny molecules capable of regulating gene expression (molecular switches) in the most critical processes for cell survival, like proliferation, cell differentiation, and apoptosis when required.[5-8]
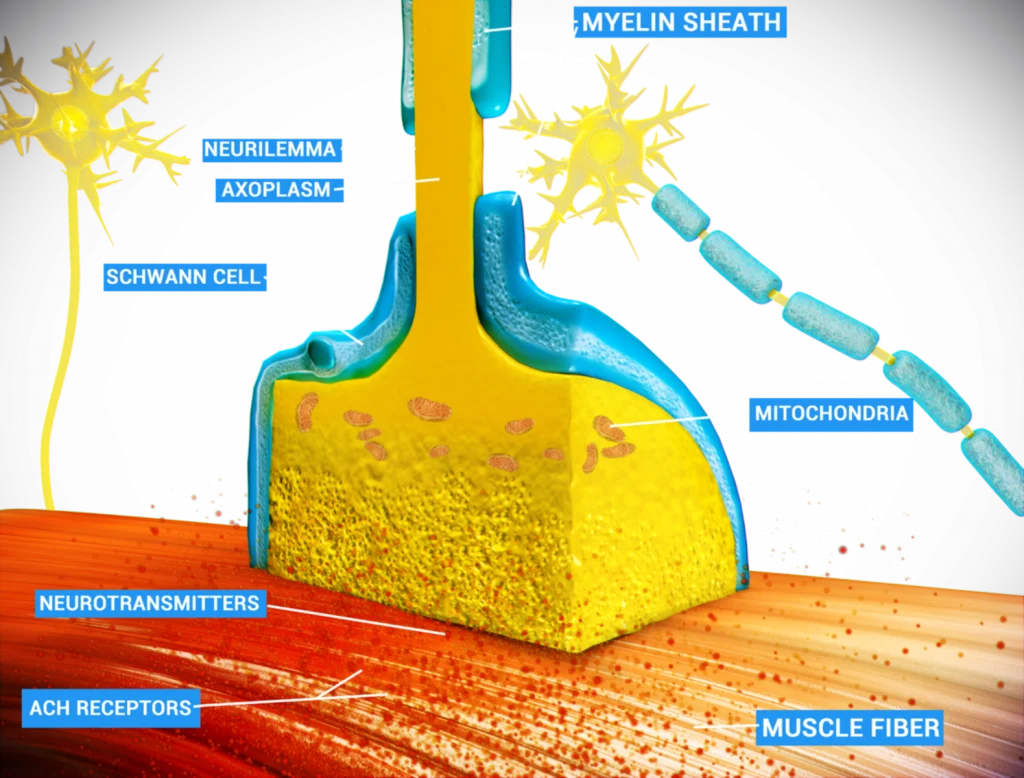
Figure 1. The comparison between the normal functioning of dopaminergic neurons in healthy individuals vs in PD patients.
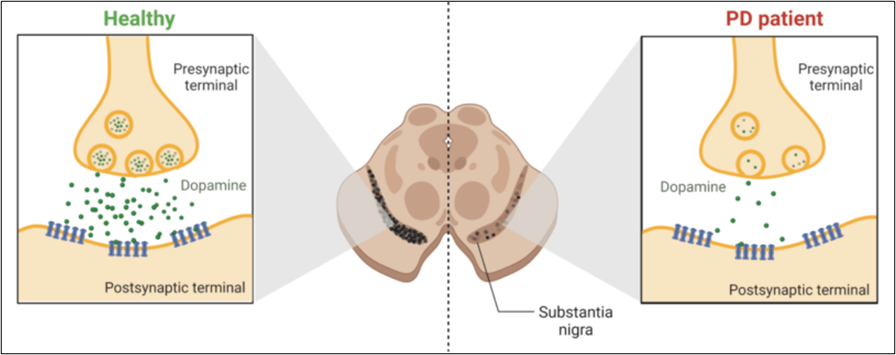
For this reason, understanding the role of miRNAs in PD could be vital to developing new treatments to decrease the progression of the disorder. The apoptosis process is significant for eliminating unwanted cells.[2] Therefore, using miRNAs as a therapy has the potential to inhibit unwanted cell death and induce apoptosis in abnormal cells as well.[3-6] Understanding this interplay between miRNAs and apoptosis could lead to new treatment strategies for PD.[7] Several investigations found that miRNAs have neuroprotection abilities, safeguarding neurons from apoptotic cell death.[5] Alternatively, miRNAs promote apoptosis, thus eliminating damaged or dysfunctional neurons, which is crucial to clear cellular debris from microenvironments.[5]
Some benefits of using miRNAs as a therapy for PD are the following: (1) miRNAs can target specific genes involved directly in the apoptotic pathway (specificity). (2) miRNAs are molecular switches, activating or inhibiting the apoptosis pathway. This adaptive characteristic is very convenient for this type of therapy since PD requires, in some cases, the inhibition of neuronal death (dopamine-producing neurons) and the activation of apoptosis in unwanted cells in the brain. (3) miRNAs can cross the blood-brain barrier and enter into cells, which is a challenge in developing neurodegenerative diseases. The apoptosis pathway accelerates the progression of PD by the direct dopamine depletion caused by the death of dopamine-producing neurons.[4-6] As the disease progresses, more and more neurons are lost, leading to worsening symptoms and disability.