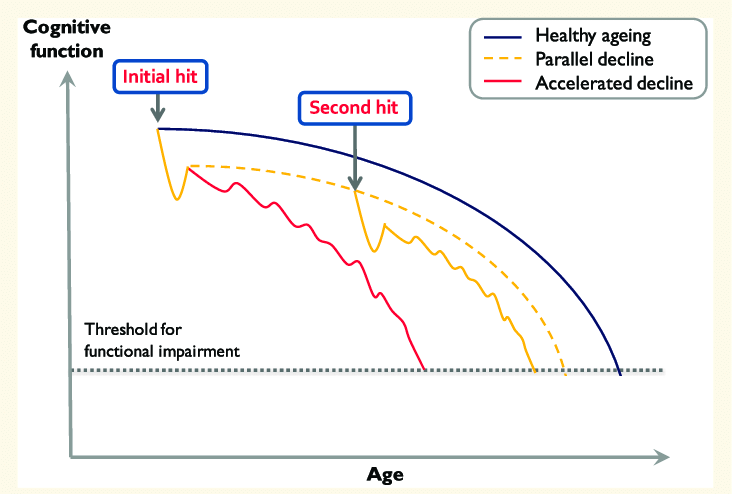
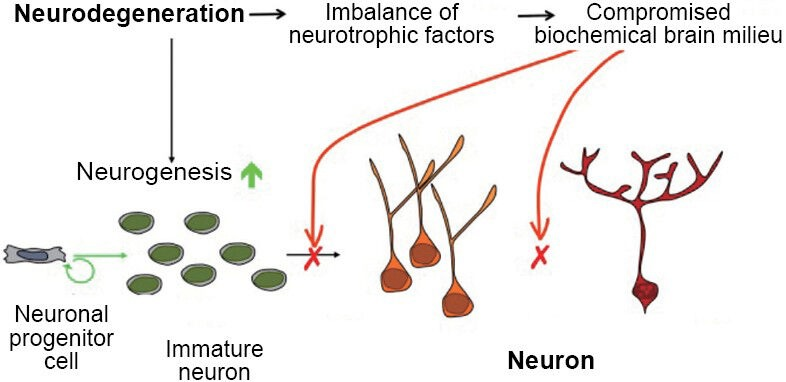
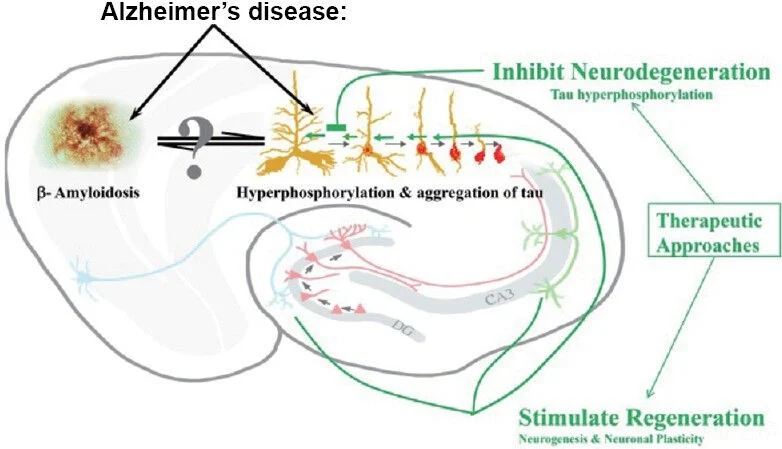
The inhibition of PU.1 using RNA therapy delivered with lipid nanoparticles as a novel treatment for Alzheimer’s disease.
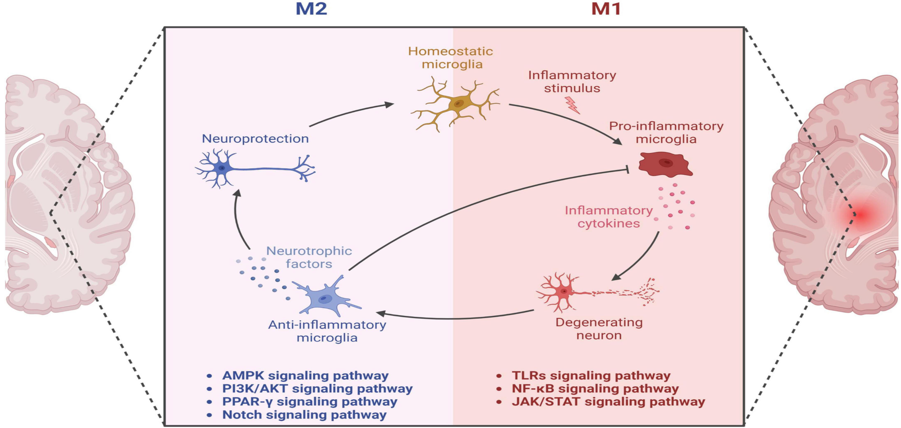
Neurodegenerative disorders are typically linked to chronic neuroinflammation. Alzheimer’s disease (AD) is not an exception since chronic inflammation is one of the hallmarks that contributes to the progression of the disorder.[2] Microglia cells are the main characters in promoting neuroinflammation since they are the most abundant brain immune cells. [2-5] Microglia cells are well known to clear different waste materials from the brain and confer neuroprotection (see Fig.1).[6] However, recent studies have pointed to the presence of AD-risk locus in the microglia genome.[2] AD-risk loci are specific fragments located in the genome that can potentially promote AD development.[3,14] These AD-risk loci open many opportunities for RNA therapeutic methods.[3,6] RNA therapies have been studied for almost all types of disorders, like Parkinson’s disease, AD, and cancer, among others.[3,9,10] The problem with this type of therapy is that it is difficult to find the correct method for transfection, depending on the area or interest. The transfection process, which introduces RNA into cells, is used to modify the host cell genome, changing the cell fate. [10,11] In the case of inhibiting with RNA transfection therapy, the siRNA is used. [2,12] siRNA (small interfering RNA) are small fragments of artificially synthesized RNA capable of inhibiting a specific genome fragment.[10]Figure 1. Show the roles of the microglia in a healthy brain versus one with Alzheimer’s disease.[6]