GHK-Cu Research
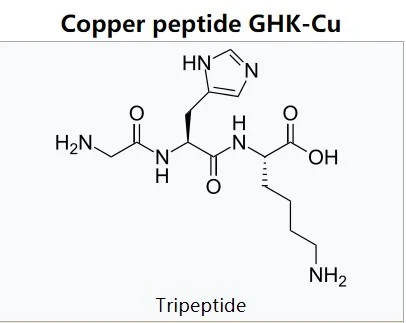
GHK-Cu is a naturally occurring peptide, made of the three amino acids Glycine-Histidine-Lysine, that is complexed with a copper molecule. It was first isolated from human plasma (a part of blood), but can also be found in saliva and urine. It has been linked to skin and tissue healing as well as to immune function and antioxidant generation. Like many natural anti-aging compounds, tissue levels of GHK-Cu tend to drop as humans age, from a high of about 200 micrograms per milliliter at age 20 to a low of 80 micrograms per milliliter by age 601.
GHK-Cu and Skin Healing
About a decade ago, research studies revealed that GHK-Cu is involved in wound healing and in the regulation of scar formation. The list of processes that research has shown GHK-Cu to be involved in includes
- Attracting cells that are involved in the repair process,
- Suppressing free radicals,
- Reducing inflammation by boosting levels of key anti-inflammatory molecules,
- Increasing protein synthesis, and
- Increasing fibroblast growth and differentiation.
Research from 2014 suggests that GHK-Cu may play an important role in regulating levels of transforming growth factor-β and insulin-like growth factor-2. By increasing levels of TGF-β and decreasing levels of IGF-2, GHK-Cu is able to improve skin healing while reducing the formation of hypertrophic scars2.
Controlled studies of GHK-Cu and aging skin in animals indicate that the peptide tightens skin, improves firmness, boosts elasticity, reduces fine lines and wrinkles, and helps to resolve photo damage. More recent research has also indicated that GHK-Cu can protect the liver from toxins, boost bone growth, and protect gastrointestinal tissue from ulcer formation. Now, it turns out, GHK-Cu also plays a role in protecting against microbial invaders.