Mesenchymal stem cell-derived exosomes as a promising therapy for Parkinson’s and Alzheimer’s Disease.
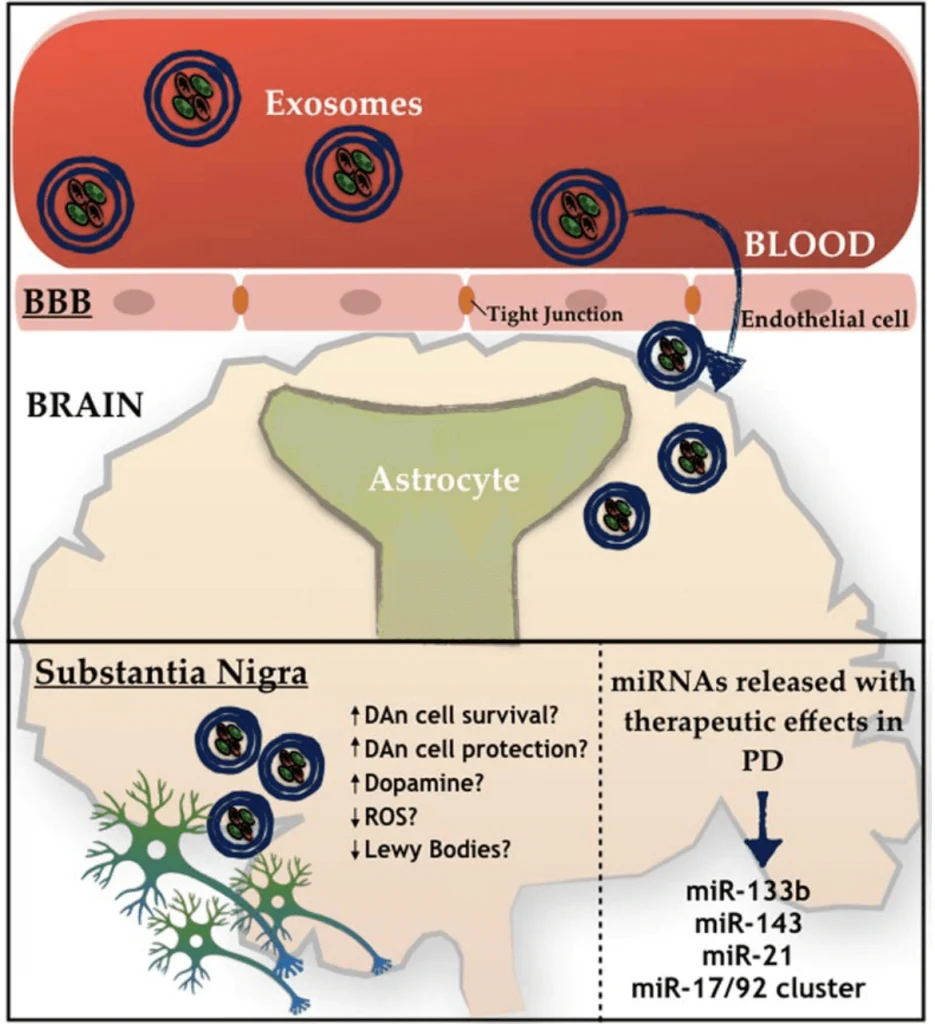
Recently, investigators suggested using Mesenchymal stem cells (MSCs)–derived exosomes as a therapy for different conditions, including Parkinson’s Disease (PD).[1,2,5] MSCs can be found in various body parts and specialize in different cell types depending on the body’s needs.[1] These cells produce extracellular vesicles called exosomes that have been studied as an alternative medicinal agent because of their stability and biological prospect in terms of the substances they carry, like signaling molecules, cytokines, enzymes, and micro-RNA (miRNA).[1,2,4] All these components are essential in maintaining cellular homeostasis, while the miRNA is more involved in regulating gene expression. [1,2,4] Many studies with MSCs have demonstrated several benefits in other neuropathological conditions. [1] One of the insights is that the MSCs have been pointed to activate different neuro-regeneration processes, opening a door for many possible ways to serve as promising therapies for future clinical trials. [1,3] Two targets for developing new treatments using MSCs are PD and Alzheimer’s disease (AD). [2,3,4,7] PD is characterized by the deterioration of dopaminergic neurons and the insufficiency of dopamine production. [3,6,9] Generally, the decrease of dopaminergic neurons is related to the accumulation of Lewy bodies (protein aggregates of α-synuclein) inside the neurons, which affects the normal functioning of those cells.[9] Interestingly, MSCs-derived exosome seems to be able to decrease one of the leading causes of PD, neuroinflammation.[2,5,10] On the other hand, AD is described as a brain illness that presents as neurological hallmarks the formation of amyloid plaques (Aβ) and neurofibrillary tangles causing synaptic loss.[4,7,12]