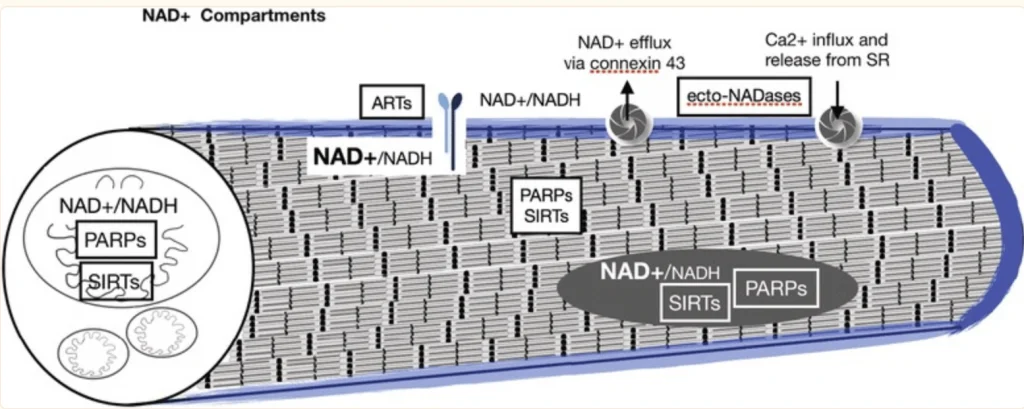
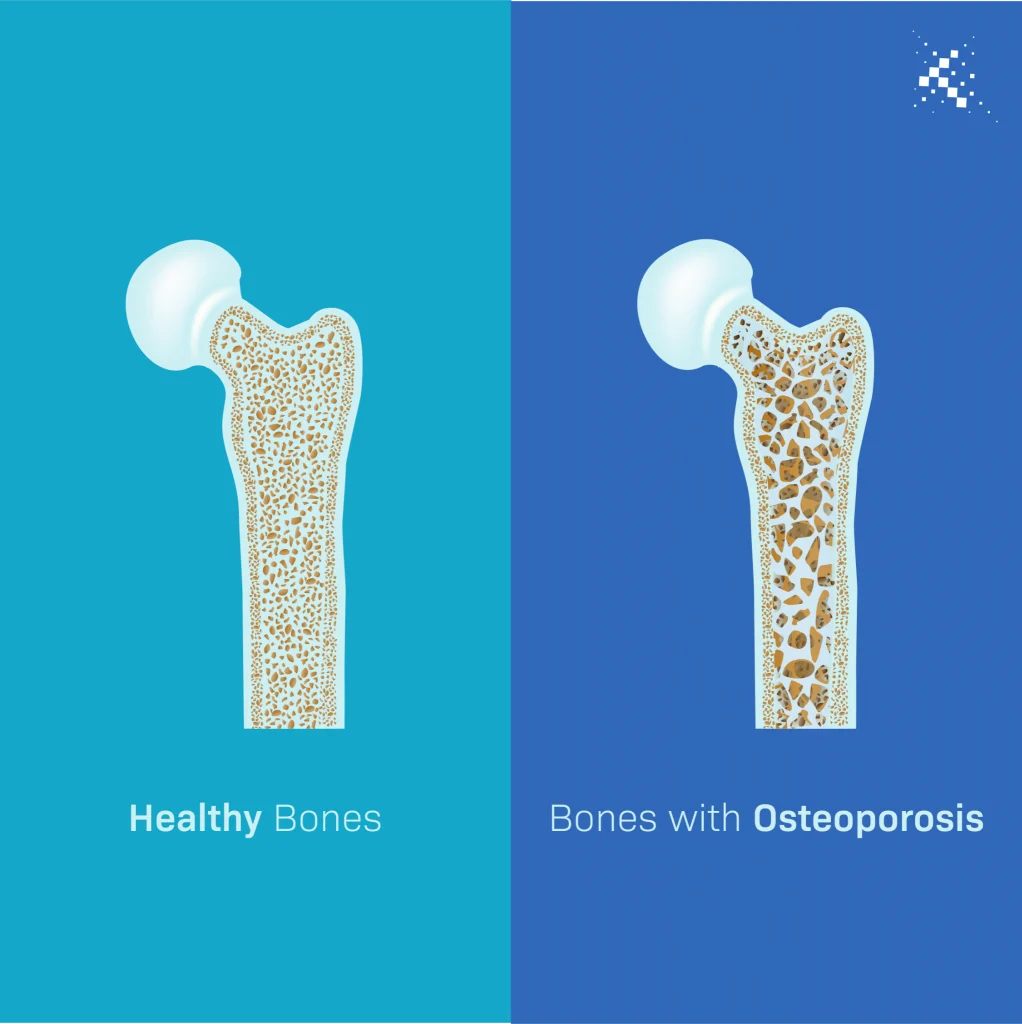
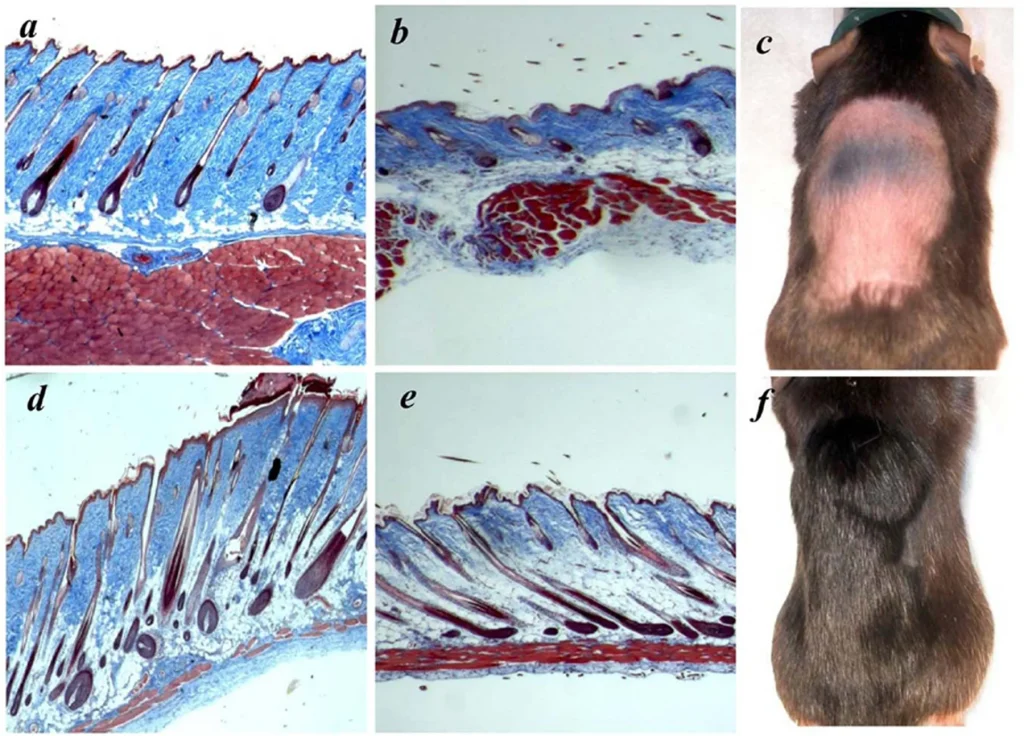
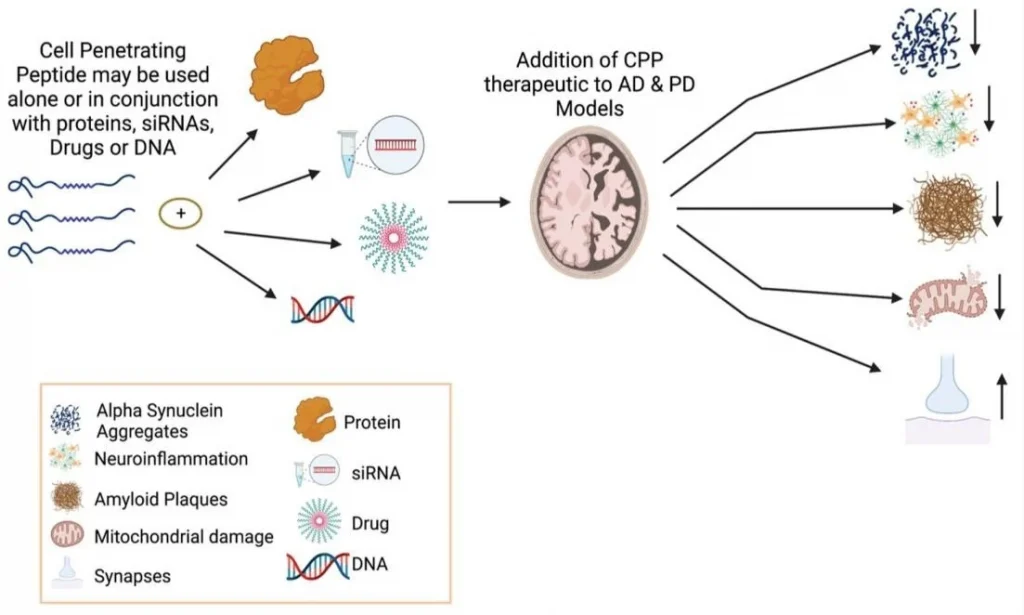
BPC-157 is a partial form of the protein known as body protection compound (BPC). BPC is a natural component within the body and has been found, in experiments on animals, to promote healing. BPC is not just active in intestinal repair and healing, but appears to produce similar effects in a number of tissues. Scientific studies based on animal test subjects has shown that its healing actions are at least partially linked to growth hormone (GH).