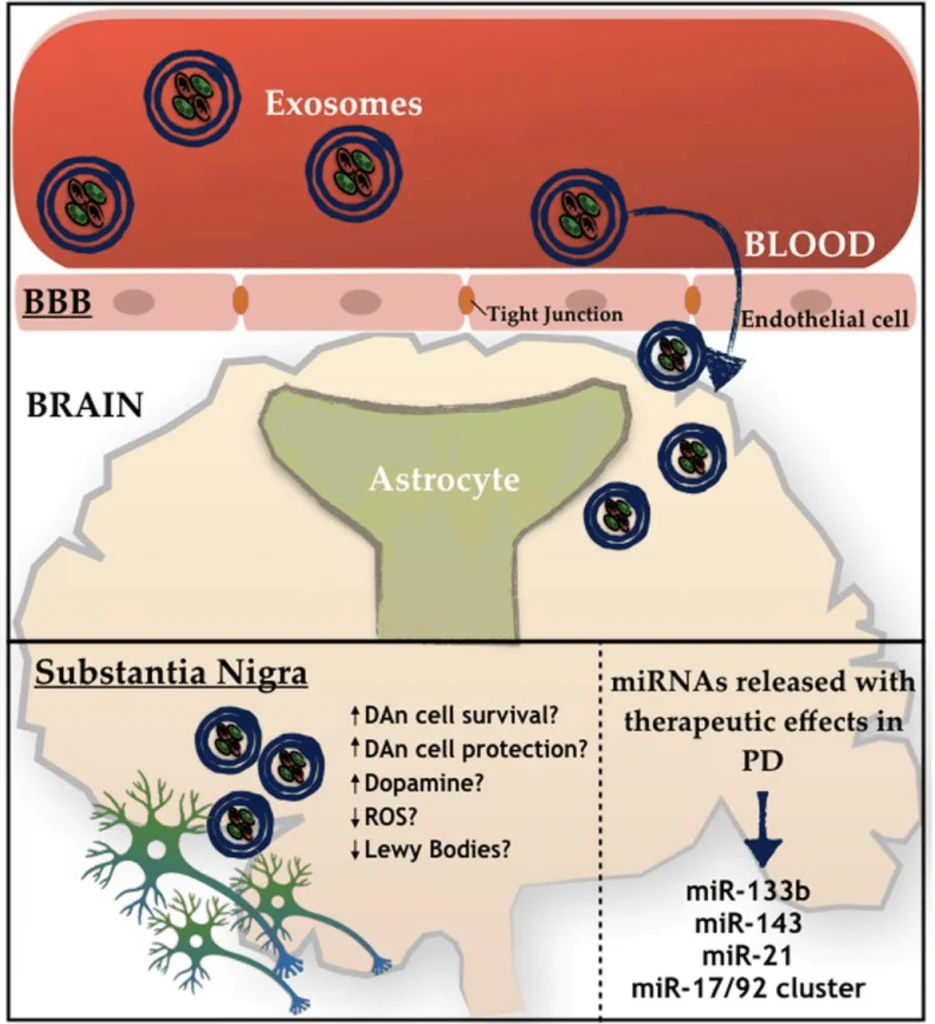
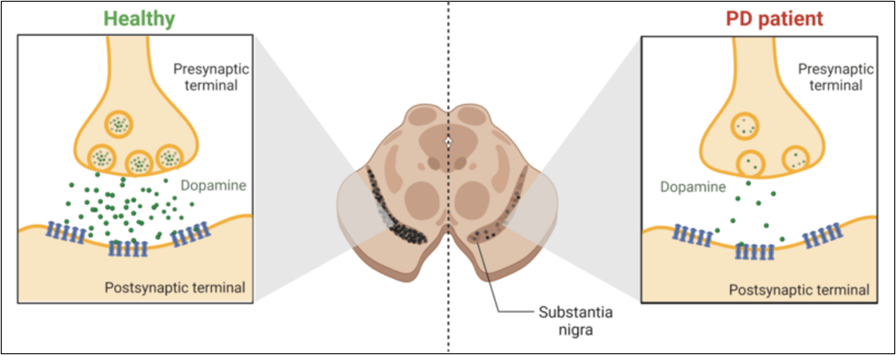
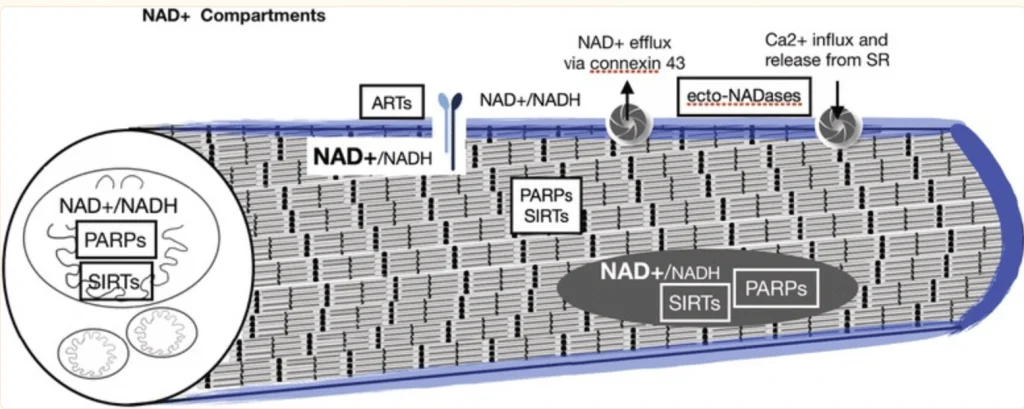
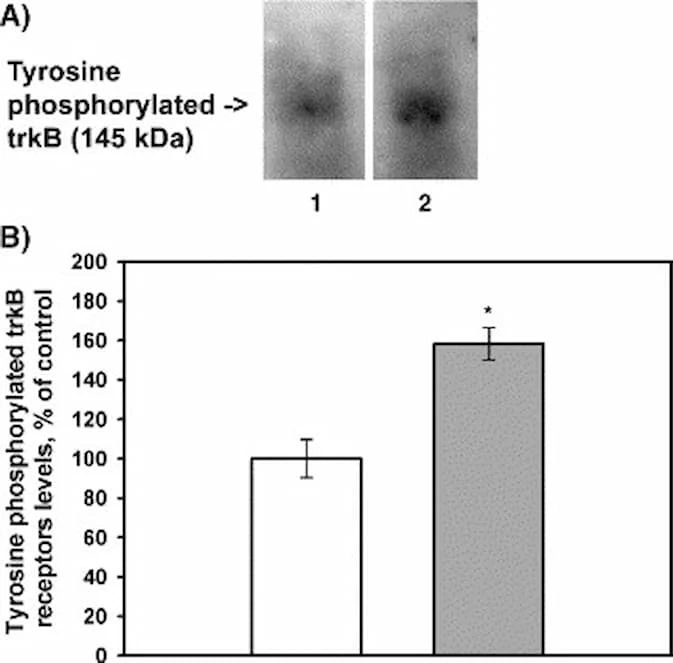
“Aging is accompanied by progressive declines in skeletal muscle mass and strength and impaired regenerative capacity, predisposing older adults to debilitating age-related muscle deteriorations and severe morbidity.
Muscle stem cells (muSCs) that proliferate, differentiate to fusion-competent myoblasts, and facilitate muscle regeneration are increasingly dysfunctional upon aging, impairing muscle recovery after injury. While regulators of muSC activity can offer novel therapeutics to improve recovery and reduce morbidity among aged adults, there are no known muSC regenerative small molecule therapeutics.
We recently developed small molecule inhibitors of nicotinamide N- methyltransferase (NNMT), an enzyme overexpressed with aging in skeletal muscles and linked to impairment of the NAD+ salvage pathway, dysregulated sirtuin 1 activity, and increased muSC senescence. We hypothesized that NNMT inhibitor (NNMTi) treatment will rescue age-related deficits in muSC activity to promote superior regeneration post-injury in aging muscle.
Results revealed that muscle stem cell proliferation and subsequent fusion were elevated in NNMTi-treated mice, supporting nearly 2-fold greater CSA and shifts in fiber size distribution to greater proportions of larger sized myofibers and fewer smaller sized fibers in NNMTi-treated mice compared to controls. Prolonged NNMTi treatment post-injury further augmented myofiber regeneration evinced by increasingly larger fiber CSA. Importantly, improved muSC activity translated not only to larger myofibers after injury but also to greater contractile function, with the peak torque of the TA increased by ~70% in NNMTi-treated mice compared to controls. Similar results were recapitulated in vitro with C2C12 myoblasts, where NNMTi treatment promoted and enhanced myoblast differentiation with supporting changes in the cellular NAD+/NADH redox states. Taken together, these results provide the first clear evidence that NNMT inhibitors constitute a viable pharmacological approach to enhance aged muscle regeneration by rescuing muSC function, supporting the development of NNMTi as novel mechanism-of-action therapeutic to improve skeletal muscle regenerative capacity and functional recovery after musculoskeletal injury in older adults.”
“NNMT inhibition using 5-amino-1MQ (30µM concentration) in both the pre-adipocytes (P < 0.01, treated pre-adipocytes vs. untreated controls) and the adipocytes (P < 0.05, treated adipocytes vs. untreated controls) resulted in significant reduction in the intracellular levels of 1-MNA…” (5)
Risk of Muscle Wasting in Elderly:
“The population of older (60+ years of age) adults is rapidly expanding in the United States and throughout the world, placing ever-increasing strains on health care resources and an urgent need for improved approaches to elder care. One of the most significant impacts of aging is the progressive decline in skeletal muscle mass and strength, with concomitant deteriorations in physical function and mobility that are strongly associated with numerous chronic diseases and increased mortality. While all older individuals experience muscle degeneration, approximately 30% of adults over 60 years of age and 50% of adults over 80 years of age develop sarcopenia, a geriatric disease characterized by significant and objective defects in muscle mass, strength, and function. Sarcopenic elderly individuals are at a 2-to 5-fold increased risk for permanent disability and greatly diminished quality of life arising from progressive muscle degeneration, decreased muscle function, and poor muscle quality that predispose them to debilitating falls and substantial disease burden. Furthermore, as muscle regenerative capacity of older adults becomes increasingly compromised, it leads to delayed and impaired recovery following muscle injury, decreased mobility and independence, increased hospitalization costs, and higher mortality rates.”